
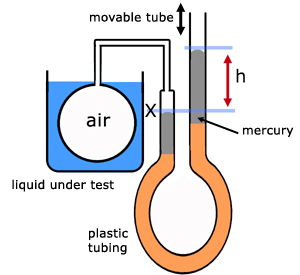

With sufficiently accurate volume measurements, this occurs to some extent for any choice of the liquid in the thermometer. Careful experiments with such thermometers produce results that deviate from Charles’ law. As we note in Section 2.8, there is a problem with this statement. Our statement of Charles’ law asserts that the volume of a gas is a linear function of the volume of the liquid in our thermometer, and that the same linear function is observed for any gas. We suppose that this thermometer uses a liquid, and we define an increase in temperature by the increase in the volume of this liquid.
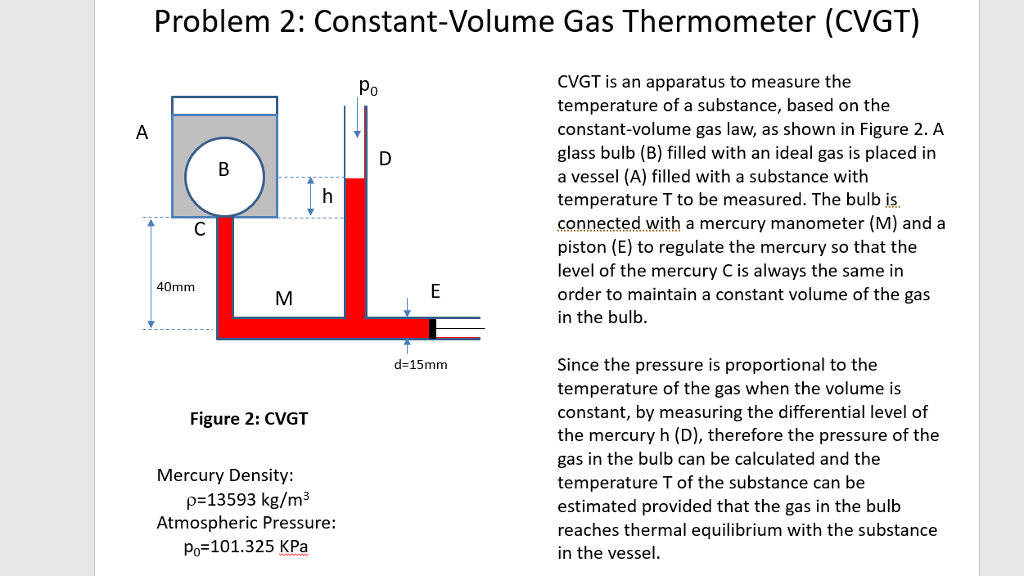
In Section 2.2 we suppose that we have a thermometer that we can use to measure the temperature of a gas.


 0 kommentar(er)
0 kommentar(er)
